Modular design: New insights into protein factories in human mitochondria
A partnership that began nearly two billion years ago still determines our lives today: A primordial cell took in a free-living bacterium as a “subtenant”. In the course of evolution, the bacterium developed into an energy specialist: With the help of oxygen, it converted ingested food into usable energy via the so-called respiratory chain. In return, it transferred other vital functions to its host cell – and even gave up part of its genetic material, which was integrated into the host cell’s genome. The former bacterium thus became a highly specialized organelle. All cells with a cell nucleus eventually evolved from this early partnership – and hence all complex living organisms, including humans.
The evolution towards higher cells was also accompanied by a major gene transfer: Most of the DNA from the former bacterial genome was integrated into the genome of the cell nucleus. Only a tiny fraction of the bacterial DNA remained in the mitochondrion, including the blueprints for key proteins of the respiratory chain.
Missing respiratory chain proteins lead to disease
These proteins are therefore produced directly in the mitochondrion by special protein factories called mitoribosomes, which are unique to this organelle. If the production of the mitoribosomes is disrupted, essential core proteins of the respiratory chain are missing, which can lead to serious, early-onset diseases.
The process of mitoribosome assembly, however, is still poorly understood – and represents a major logistical and coordinative challenge for the cell. This is because the assembly instructions for the basic building blocks of the mitoribosomes – the ribosomal RNA and 82 ribosomal proteins – are encoded in two different genomes. The former is encoded in the mitochondrial genome, the latter in the nuclear genome. This has implications for ribosomal protein production. The mitoribosomal proteins are produced in the cytoplasm and must then be imported into mitochondria.
Roadmap for ribosomes in human mitochondria
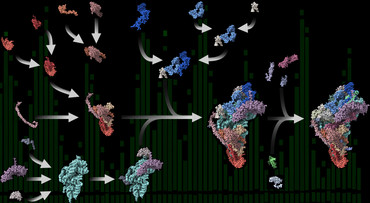
Research teams led by Ricarda Richter-Dennerlein at the University Medical Center Göttingen (UMG), as well as Juliane Liepe and Henning Urlaub at the Max Planck Institute (MPI) for Multidisciplinary Science, have now provided the first comprehensive roadmap of human mitoribosome assembly – from very early to late steps. The scientists found out that this process is surprisingly modular.
“The intermediate complexes formed during the mitoribosome production are very small and highly dynamic. Therefore, they are difficult to study biochemically or to observe directly during their assembly,” reports Richter-Dennerlein, group leader and professor at the UMG and member of the Cluster of Excellence “Multiscale Bioimaging: From Molecular Machines to Networks of Excitable Cells” (MBExC). Applying a multidisciplinary approach was key to following the entire assembly pathway of human mitoribosomes. Using biochemical experiments, the scientists were able to isolate and analyze mitoribosomal assembly complexes. To reconstruct the process step by step, they combined the obtained data with results from quantitative mass spectrometry experiments contributed by Urlaub’s group at the MPI and the UMG, as well as data obtained through mathematical modeling by the team of research group leader Liepe at the MPI. “In a great team effort, we were finally able to create an almost complete map of human mitoribosome assembly,” says Richter-Dennerlein.
Modular assembly
The researchers discovered that the ribosomal proteins produced in the cytoplasm are imported into the mitochondria, where they are assembled into protein-only modules. “These modules are produced in large quantities and are thus available in excess,” Liepe explains. “They then assemble in a coordinated fashion on the appropriate ribosomal RNA moiety to form mitoribosomes in the organelle with the help of special factors.” Like ribosomes in bacteria or the cell’s cytosol, the functional mitoribosome consists of two subunits of different sizes.
Elena Lavdovskaia, first author of the paper now published in the journal Nature Structural & Molecular Biology, adds: “The data suggest that ribosomal RNA production limits mitoribosome assembly. When mitochondria produce too little ribosomal RNA, mitoribosome formation stops. Our experiments therefore also explain how mitochondria cope with the challenge of forming a protein factory of dual genetic origin.”
The scientists hope their findings will lead to a better understanding of how the assembly and disassembly of the mitochondrial protein factory takes place and how disruptions occur that cause disease.
Original publication: https://doi.org/10.1038/s41594-024-01356-w
CONTACT
Max Planck Institute for Multidisciplinary Sciences
Press Officer and Head of the Communications & Media Team
Carmen Rotte (Press contact MPI-NAT)
Am Fassberg 11, 37077 Göttingen
Phone +49 551 / 201-1304
carmen.rotte(at)mpinat.mpg.de
University Medical Center Göttingen, University of Göttingen
Corporate Communication
Lena Bösch (Press contact UMG)
Von-Siebold-Str. 3, 37075 Göttingen
Phone +49 551 / 39-61020, Fax +49 551 / 39-61023
www.umg.eu